Chemical Properties of Wood: Amazing Wood Chemistry
As a wood enthusiast, the allure of wood lies not only in its aesthetic appeal but also in its chemical complexity. While I might not be a chemist, my extensive studies have opened a realm of chemical interactions within wood. This understanding is vital, offering insights into wood’s behavior, constituents of wood, treatment, and overall significance of chemical analyses in our lives.
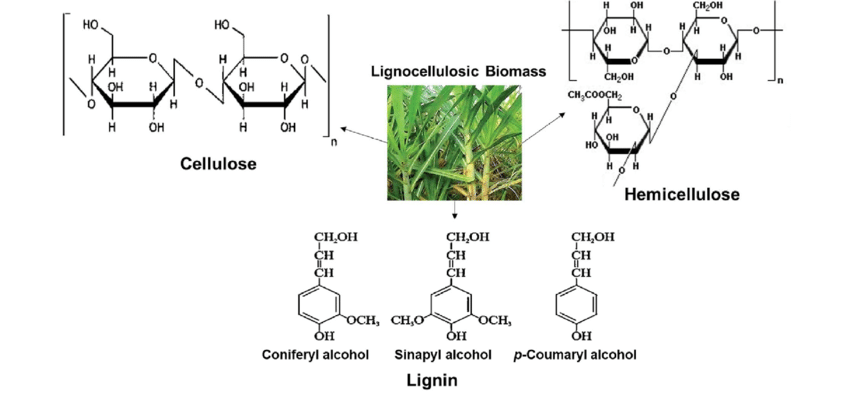
Following are the wood chemical constituents at a glance.
Dry Wood:
- Cellulose: 40 – 50% | Primary structural component of the cell wall.
- Hemicellulose: 20 – 30% | Polysaccharide that provides flexibility.
- Lignin: 15 – 30% | Provides rigidity and resistance against microbial attack.
- Wood Extractives: 1 – 5% | Encompasses a variety of compounds including resins, oils, and tannins.
- Minerals: 0.1 – 1% | Inorganic components primarily found in the cell wall. These are calcium (Ca), potassium (K), magnesium (Mg), manganese (Mn), iron (Fe), sodium (Na), zinc (Zn), aluminum (Al).
Green Wood:
The relative percentages in the green wood (assuming a moisture content of 50%) would be:
- Water: 50%
- Cellulose: 20 – 25%
- Hemicellulose: 10 – 15%
- Lignin: 7.5 – 15%
- Wood Extractives: 0.5 – 2.5%
- Minerals (Ash): 0.05 – 0.5% | These are calcium (Ca), potassium (K), magnesium (Mg), manganese (Mn), iron (Fe), sodium (Na), zinc (Zn), aluminum (Al).
I will go through a detail of the wood behavior due to chemicals here.
Polymeric Components of Wood (The Main Chemicals)
Cellulose
Cellulose, the primary structural component in plants and the most abundant organic polymer on Earth, has always been a point of fascination for me. Accounting for approximately 40-50% of wood’s composition, cellulose plays a pivotal role in defining the properties of different wood types and wood growth.
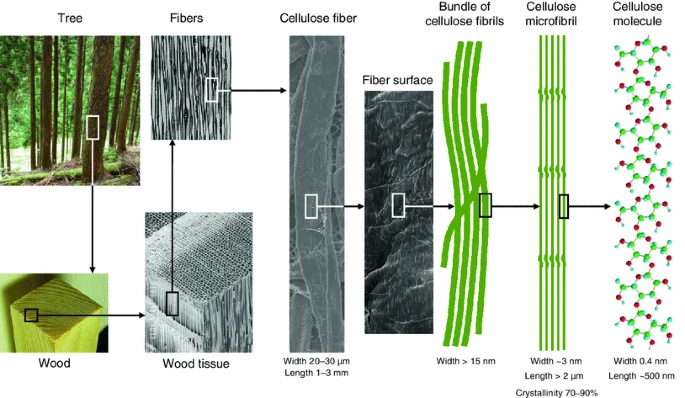
Structure and Composition
- Composed of complex carbohydrates.
- Linear chains of β-D-glucose units.
- Connected by β(1→4) glycosidic bonds.
- These chains bundle together forming:
- Hydrogen-bonded microfibrils.
- Highly resilient structural units.
- Degree of polymerization often exceeds 10,000, explaining wood’s resilience.
Role in Wood
- Bestows tensile strength and rigidity to wood.
- The primary reason trees can stand tall against nature’s forces:
- Harsh winds.
- Heavy snow.
- Environmental pressures.
- Historical significance:
- Made wood a favorite building material through the ages.
- From ancient shelters to modern skyscrapers.
Hemicellulose (Polysaccharides)
Hemicellulose constitutes around 20-35% of the wood’s dry weight, with the percentage varying depending on the species. Distinct from the homopolymeric cellulose, hemicelluloses showcase a heteropolymeric structure, crafted from an array of sugar monomers. While often overshadowed by cellulose, the role hemicellulose plays in the intricate matrix of wood cell walls is nothing short of indispensable.
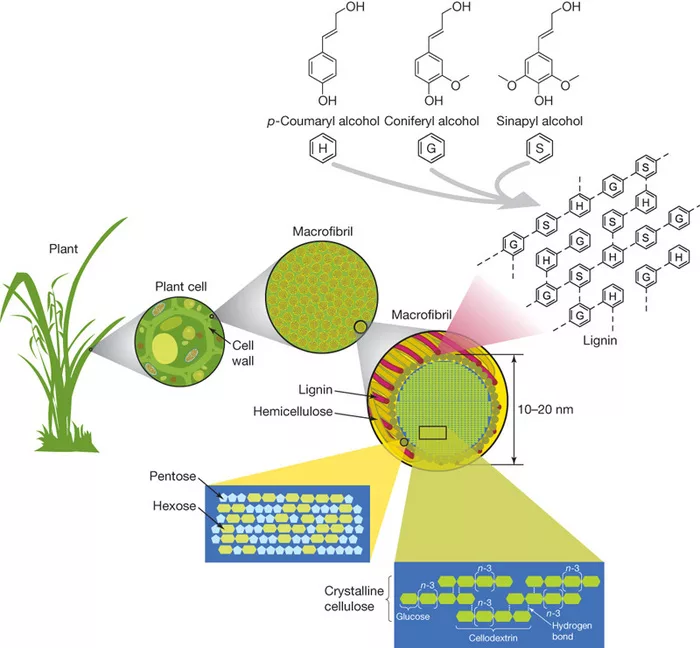
Diversity of Sugars
- Hemicelluloses are composed of various pentoses (5-carbon sugars) like xylose and arabinose, and hexoses (6-carbon sugars) such as mannose, glucose, and galactose.
- Softwoods typically have a higher content of mannose, making up the backbone of galactoglucomannans.
- Hardwoods, on the other hand, contain predominantly xylans, with xylose as the major sugar component.
- These sugar units impart unique properties to the wood, influencing factors like porosity, absorbency, and even color.
Interaction with Cellulose
- Hemicelluloses function as the “glue” in wood, binding cellulose fibrils together.
- This interaction plays a pivotal role in determining the porosity and reactivity of wood.
- The intertwined nature of cellulose and hemicellulose provides mechanical strength, flexibility, and resistance to various stresses.
- This matrix also influences how wood reacts to treatments like pulping, with hemicellulose often being more susceptible to chemical breakdown than cellulose.
Lignin
Diving deeper into my wood research, I came across the significance of lignin. Comprising about 15-30% of wood’s composition, lignin composition plays a crucial role in safeguarding wood’s structural integrity and ensuring its longevity. Lignin, acts as the glue, binding cells and fibers together.
Composition and Variability
- Lignin is a distinguished polymer with an intricate aromatic structure.
- Its composition in wood varies significantly: around 18% in softwoods like pine to nearly 30% in certain hardwoods.
- This compositional difference is a key factor in the distinct characteristics and appearance of each wood species.

Significance in Wood
- Lignin serves as a primary binder in wood, providing cohesive strength.
- It acts as wood’s natural armor, defending against microbial decay.
- This protective function allows trees to stand resiliently for centuries, enduring environmental threats and the test of time.
- Did you know? Bamboo, which isn’t technically a tree but a grass, has a lignin content of about 20-25%, contributing to its strength and flexibility.
Extractives – Nature’s Unique Recipe
From my early woodworking days, I was captivated by the way wood released its aroma, which often reminded me of my childhood walks in the forest.
Organic Extractives – Nature’s Chemical Elixirs
Resin Acids and Fatty Acids
- These compounds offer woods like pine their unique, nostalgic scent while acting as a frontline defense against pests.
- I recall using pine for a project, and as I worked, its aroma enveloped my workspace, transporting me back to nature.
Terpenes and Terpenoids
- These compounds, found abundantly in woods like cedar, gift us with the soothing fragrance we associate with wooden chests or closets.
- Did you know that the pleasant smell of freshly cut cedarwood is largely due to its terpene content?

Other Notable Organic Extractives: Tannins, steroids, lignans, and alkaloids
- These might not be the stars of the show, but their presence adds depth to wood’s protective and aesthetic qualities.
- Tannins, for instance, are what make certain woods excellent for wine barrels, as they contribute to the wine’s flavor profile.
- The right solvent is essential for extracting specific compounds.
Inorganic Extractives – The Subtle Contributors
Minerals
- These trace elements, although overlooked, can subtly affect a wood’s hue and durability. For instance, woods with higher mineral content often showcase unique grain patterns.
Silica and Sulfur
- Their presence, while not dominant, can be critical for woods that thrive in challenging environments, bolstering their natural resistance.
- Fun fact: Bamboo, technically a grass but often discussed in woodworking circles, has high silica content, giving it its remarkable strength.
Water in Green Wood: A Pivotal Chemical Aspect
Water, frequently overlooked in wood’s chemical conversations, is paramount in influencing the timber’s characteristics both chemically and physically. Water’s integration in wood transcends mere physical presence; it’s a dynamic participant in the wood’s chemical narrative, influencing its properties and interactions at every stage.
Chemical Role in Living Trees
Within living trees, water isn’t just a physical presence; it acts as a solvent and medium for chemical reactions, aiding nutrient transportation and metabolic processes.
Dissolution and Transport
Many of the tree’s essential nutrients are soluble in water, enabling their transport through the tree. This solubility plays a fundamental role in the tree’s biochemistry.
From Green to Seasoned Wood: A Chemical Transition
Harvested timber, termed “green” due to its moisture content, undergoes chemical changes during drying.
Impact on Chemical Bonds
Drying alters the hydrogen bonding between wood’s molecules, leading to physical changes like shrinkage. The removal of water can also influence other chemical bonds and interactions within the wood.
Resistance and Durability
The reduction of water decreases the wood’s susceptibility to chemical degradation from enzymes and microbes, enhancing its resistance to decay.

Chemical Bonding (Vital Chemical Properties of Wood)
Types of Bonds in Wood
Understanding the chemical bonding in wood is pivotal to appreciating its sturdiness and adaptability. Diverse bond types in wood contribute not only to its mechanical strength but also to its dynamic interactions with the environment.
Hydrogen Bonding
- Approximately 2.5-3.5 hydrogen bonds form per cellulose molecule in wood, playing a critical role in its structure.
- These bonds are fundamental to wood’s hygroscopic nature, dictating how it absorbs moisture from the air, which can be up to 20% of its weight in certain conditions.
- Hydrogen bonding also greatly impacts wood’s dimensional stability, affecting how it swells and shrinks with moisture changes. See, how chemical properties influence its mechanical and physical properties!
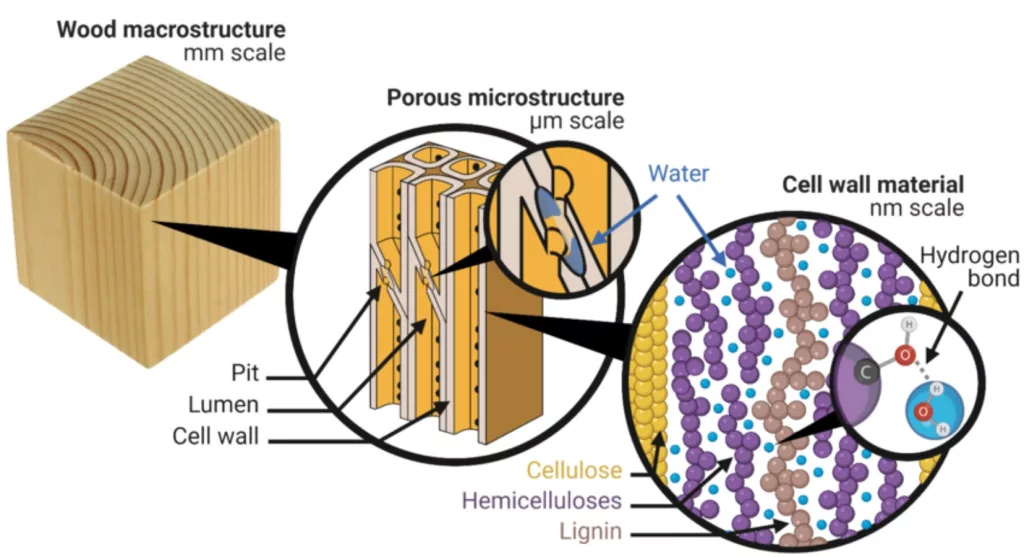
Covalent and Ionic Bonding
- Covalent bonds, being the strongest, are responsible for holding atoms together within the wood’s molecular structure. This robust binding contributes to wood’s inherent tensile strength.
- While less prevalent than covalent bonds, ionic bonds also exist in wood, especially in certain reactions or when wood undergoes specific treatments.
- The presence of these bonds ensures wood’s ability to react with various chemicals, enabling processes like pulping in paper manufacturing or chemical modifications for enhanced durability.
To put a number on it, the vast majority (well over 95%) of the chemical bonds in wood would be covalent in nature.
Specifically:
- Cellulose: This is a polysaccharide made up of glucose units linked by β-1,4-glycosidic bonds, which are covalent in nature. Cellulose chains are held together by hydrogen bonds (which are not truly ionic but are dipole-dipole interactions).
- Hemicellulose: These are branched polysaccharides that contain various sugar monomers. Like cellulose, the main connections in hemicellulose are covalent glycosidic linkages.
- Lignin: Lignin is a complex aromatic polymer. Its structure involves various types of covalent bonds, including ether linkages and carbon-carbon bonds. As a portion of wood’s overall composition, these ionic interactions are minor in comparison to the covalent nature of the primary constituents.
Chemical Modification Potential – Unlocking Wood’s Superb Chemical Properties Via Chemical Treatment
Wood’s chemical composition opens the door for a variety of modifications. By tinkering with its inherent properties, scientists and craftsmen have been able to cater to specific needs, enhancing its usability.
Common Modifications
Across the vast expanse of wood science literature I’ve delved into, it’s evident that certain chemical modifications can significantly elevate wood’s natural attributes.
Acetylation
- Through acetylation, wood’s hydroxyl groups are transformed, reducing its ability to absorb water by nearly 80%.
- This process extends the wood’s lifespan, making it particularly suited for regions with high humidity, with some acetylated woods lasting up to 50 years without significant degradation.
Furfurylation
- Furfurylation, a process involving the impregnation of furfuryl alcohol, can increase wood’s decay resistance by up to 10 times compared to untreated wood.
- It’s a preferred treatment for woods used in outdoor settings, helping structures withstand the test of time and natural decay.
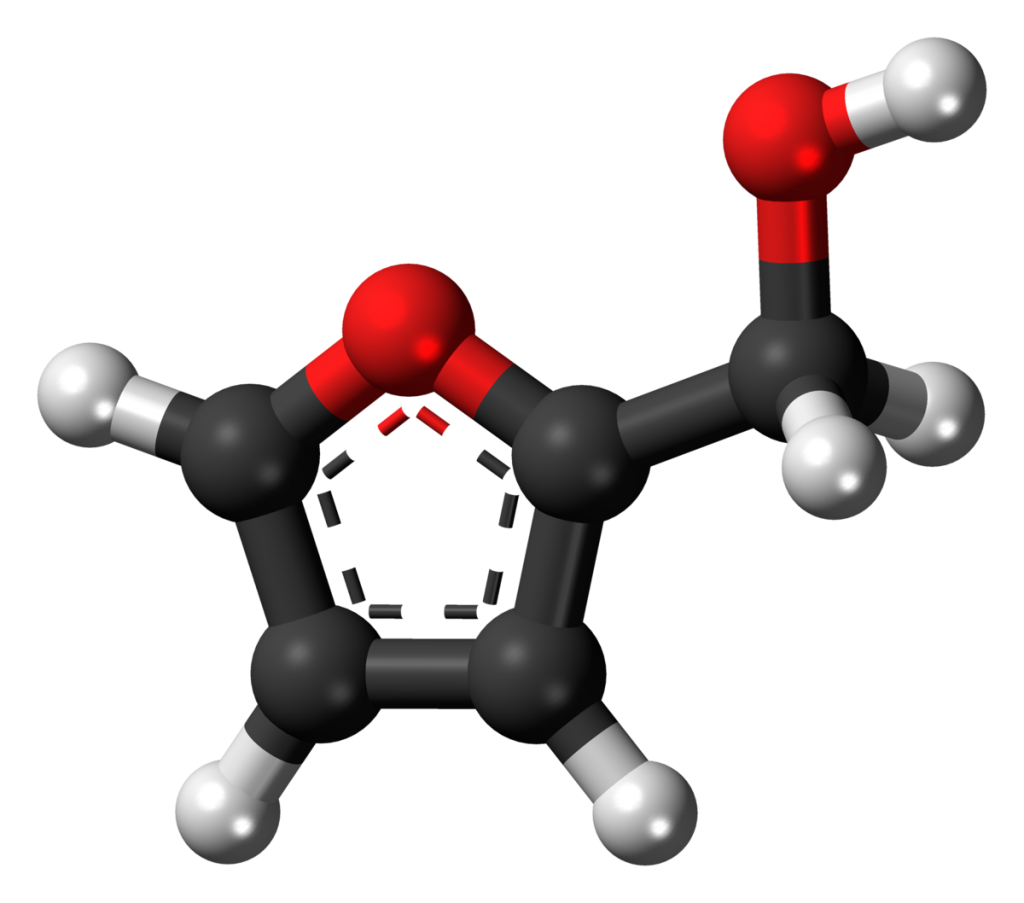
Esterification and Hydrolysis
- Esterification involves replacing wood’s hydroxyl groups, enhancing its resistance against moisture and microbial attack. Hydrolyzed woods, on the other hand, can show an increased resistance to UV degradation.
- These treatments can elevate the wood’s resistance metrics by 30-40%, safeguarding it against various environmental adversaries.
Chemical Processing of Wood – Humanity’s Artful Hand on Nature’s Canvas
Our ancestors quickly recognized the potential of wood, manipulating it to craft tools, shelter, and art. Today, with advanced chemical processes, we’ve further unlocked its potential.
Transcending Nature: Wood’s Man-Made Evolution
Ancient civilizations, like the Egyptians, used oils and resins to preserve wood, a primitive yet effective form of chemical processing. Today’s treatments, ranging from pressure treating to advanced polymer infusions, are just the modern continuations of this age-old practice.
Behind the Scenes of Timber Transformation
- Heat treatment of wood, a process I’ve personally worked with, changes its color and enhances its resistance to decay. This simple process, without the addition of any chemicals, can significantly extend the wood’s life.
- Another transformation, the acetylation of wood, involves changing the molecular structure to make it more resistant to moisture. It’s amazing to think that small tweaks at the molecular level can create a product with entirely new properties!
- Fun fact: Some of the oldest wooden artifacts, like the 5,000-year-old wooden statues found in Egypt, have endured through time due to the region’s dry climate and perhaps some primitive forms of chemical preservation.

Interactions with Additives and Agents – Crafting with Precision
Anyone who’s ever crafted with wood knows the significance of how it interacts with external agents. These interactions can transform ordinary wood into extraordinary masterpieces.
Adhesion and Binding
Venturing into woodworking, I’ve learned that the strength of wooden products is just as much about what binds them as the wood itself.
Adhesion with Glues and Resins
In 1912, the first synthetic, non-toxic wood glue, PVA, was discovered. This marked a revolution in how wood items, from artful sculptures to pragmatic furniture, could be held together. Ensuring sturdy bonds means wooden artifacts can often outlive their creators.
Dye and Stain Uptake
Dyes and stains don’t just color wood; they tell its story. Pine, for instance, is known to produce different hues based on the type of stain due to its uneven grain. The right stain can make a piece look antique, modern, or somewhere in between.

Uptake and Binding of Preservatives
I was surprised to learn that some ancient wooden structures, like the 1416-year-old Horyu-ji Temple in Japan, owe their longevity to early wood preservation techniques. Modern preservatives can extend wood life by decades, if not centuries, allowing for legacies to be built.
Volatile Emissions
Who hasn’t been captivated by the comforting scent of fresh-cut wood? It’s more than just a pleasant aroma; it’s a dance of compounds interacting with the air.
Volatile Organic Compounds (VOCs)
Different woods release varying VOCs. Cedarwood, for example, emits a scent ( or chemical content) that has been cherished for centuries and is even believed to repel insects. However, some VOCs can affect indoor air quality, a concern in modern, tightly-sealed homes.
Anisotropic Variations in Wood
The way wood behaves isn’t random; it’s a testament to its growth and the trials it faced throughout its life.
Directional Variations
I’ve been astounded by how the same piece of wood can display different strengths based on its cut. This uniqueness is because wood, unlike metals and plastics, has direction-dependent properties.
Radial, Tangential, and Longitudinal Variations
When crafting, these orientations are paramount. Radial cuts, for instance, often result in tighter grain patterns and are less prone to warping compared to tangential ones. This directional dance dictates everything, from a wood’s drying rate to its final gleam when polished.
Minor Components and Specific Properties – The Hidden Mysteries of Wood
Wood, a material so commonly used, still holds enigmas that can astonish even seasoned wood enthusiasts like myself. I recall a time when I stumbled upon a century-old oak, its majestic beauty captivating my senses. To my surprise, the seasoned woodworkers in the area mentioned how the wood from such older trees had unique characteristics because of their starch and protein makeup, which differed from younger trees.
Lesser-known Constituents – The Unsung Heroes
Beyond the familiar trio of cellulose, hemicellulose, and lignin, there are other hidden warriors in wood. These components might be lesser-known but play pivotal roles that often go unrecognized.
Proteins
- Wood’s protein content stands less than 1% of its dry weight.
- These proteins aren’t just elements; they’re crucial to a tree’s lifecycle, aiding growth, defense, and repair.
- It’s fascinating to think of them as the silent sentinels, ensuring trees remain robust across varied environments.
Starch
- A tree’s energy bank, starch, has often been a topic of conversation among wood enthusiasts. This is Complex carbohydrates composed of simple sugars.
- It’s a fun fact that one can gauge when a tree was chopped by examining its starch content; spring and summer usually present higher amounts.
- I’ve personally seen how woods with higher starch levels become a haven for pests :), a testament to nature’s food cycle.
- The adaptability of wood, particularly in terms of treatment, can be linked back to its starch content, adding yet another dimension to our understanding of this versatile material.
Specialized Chemical Properties of Wood – Delving Deeper into the Wood’s World
Diving deep into the chemistry of wood has always been a journey of discovery for me. While the general properties of wood are widely understood, it’s these specialized chemical aspects that truly underscore its complexity and versatility.
Redox Potential and Reactions
- Wood maintains a dynamic relationship with its environment, leading to oxidation or reduction reactions under specific exposures.
- A personal experience brought this to light when an oak piece turned gray due to its exposure to iron and tannins – a clear redox reaction.
- A notable example is black walnut wood. Its natural oxidation results in its cherished deep color, making it popular for furniture.
Sorption Behavior
- The interplay between wood and atmospheric moisture is intricate. Sorption isotherms graphically represent this, detailing moisture absorption relative to ambient humidity.
- An enlightening experience was when a woodworking piece unexpectedly expanded in a humid summer. This incident emphasized the significant role of environmental conditions in woodworking.
Diffusion and Permeability
- Fluid transport in wood is determined by its inherent porous nature. Permeability can drastically vary.
- Industries often utilize the permeability of woods like pine for pressure treatments, driving chemicals deep to augment decay resistance.
- A personal experiment using a denser wood for deep staining didn’t yield the desired result, underscoring the need to match wood characteristics to specific applications.
Compatibility and Composites – Bridging Natural and Synthetic Worlds
Venturing into the sphere of wood-polymer composites was an enlightening transition in my journey with wood. The blend of organic and synthetic materials has ushered in a new era of possibilities, opening doors to applications I hadn’t fathomed.
Wood-Polymer Interactions – Crafting the Future
When wood, with its intricate cellular structure, meets synthetic polymers, the results can be both groundbreaking and unpredictable. These interactions are driving the evolution of new-age materials that harness the best of both worlds.
Compatibility with Polymers
- The compatibility between wood and polymers varies. For instance, wood flour-filled polypropylene composites can have wood content ranging from 30% to 70%.
- The key is understanding the interplay at the molecular level. For instance, ensuring that the polymer encapsulates the wood particles properly is essential to achieve desired mechanical properties.
- I recall an experiment where I attempted to blend wood fibers with a particular polymer. Initially, the results were less than stellar. However, after tweaking the ratio and using a specific coupling agent, I achieved a composite that was not only durable but also aesthetically pleasing. This experience underscored the importance of understanding wood-polymer interactions at a granular level.

Reactivity and Interaction – Understanding Wood’s Nature
Wood and Moisture – A Delicate Dance
Hygroscopicity
- Wood’s inherent ability to absorb or release moisture can dictate its behavior. This trait requires careful consideration, especially in fluctuating climates.
Dimensional Stability
- Ensuring a wooden piece retains its dimensions over time is essential. I’ve seen many a masterpiece warp due to neglecting this critical aspect.
Chemical Reactivity – Wood’s Ever-Adapting Persona
Acidity and Alkalinity (pH)
- Different woods have varying pH levels that varies along with age, region, soil type, area of the wood in the tree, etc., which can influence decisions when selecting adhesives or finishes.
Interaction with Chemical Agents
- Being mindful of wood’s chemical reactions is key. Once, I mistakenly applied a certain finish to mahogany, only to watch it react and change its color unexpectedly.
Flammability and Combustion – Playing Safe with Wood
Factors Influencing Flammability
- It’s essential to know which woods are more susceptible to fire, especially when crafting items for households.
Fire Preventing Chemical Treatments
- These are the unsung heroes that enhance wood’s fire resistance. Such treatments can make the difference between a structure lasting decades or being consumed by flames. It changes the chemical behavior of the woods forever.
Biological Resistance and Degradation – Nature’s Tug of War
As I’ve delved deeper into the intricacies of wood, it’s always amazed me how a seemingly passive material is so actively engaged in its own defense and survival.
Defense Against Microorganisms – The Great Wall of Wood Natural Resistance

- Some woods, like cedar and teak, are naturally resistant to termites and fungi due to the presence of certain organic compounds. For instance, teak contains a high oil content, around 40-50%, that acts as a natural repellent against pests.
- From personal observation, I’ve found that naturally resistant woods tend to have a longer lifespan, even in challenging environments.
Chemical Treatments
- Pressure-treated lumber, for instance, can be fortified to last up to 40 years or more against termites and rot. These treatments work by infusing the wood with chemicals, increasing its resistance to various pests.
- An interesting fact: The greenish tint seen in pressure-treated wood is due to the copper-based preservatives used to enhance its durability.

Degradation and Stability – Battling Time and Elements
Thermal and Photodegradation
- Direct exposure to sunlight can cause wood to lose its color, a process accelerated by UV rays. For instance, untreated pine can turn from a vibrant yellow to a dull gray after just a few months of direct sun exposure.
- I once built a wooden bench for my garden without considering photodegradation. Within a year, it had lost much of its original charm due to color fading. A hard lesson on the importance of protective coatings!

Oxidative Stability
- Oxygen, when combined with certain environmental factors, can degrade wood. Hardwoods, due to their dense cell structure, often exhibit better oxidative stability than softwoods.
- A tidbit from history: Many ancient wooden artifacts, preserved for centuries, owe their longevity in part to their oxidative stability, a testament to wood’s enduring nature.
Water and Wood’s Equilibrium Moisture Content (EMC)
Beyond just a physical balance, EMC is a state where chemical stability is achieved within the wood, minimizing potential reactions due to moisture fluctuations.
Direct Chemical Interactions
Water engages in direct chemical reactions within wood, especially when in contact with wood’s primary components.
Hydrolysis
Certain conditions facilitate the hydrolysis of wood’s components, breaking chemical bonds and potentially weakening the timber.
Extractive Solubility
Water’s solvent properties can dissolve certain wood extractives, potentially altering the wood’s color, aroma, and protective attributes.
Chemical Properties’ Impact on Physical Properties of Wood – The Hidden Alchemy
Every touch, every step on a wooden surface, every carved masterpiece – they all derive their essence from the unseen chemistry within.
When Chemistry Meets Texture – Crafting Nature’s Tapestry
Did you know that the oak’s renowned strength is in part due to its high tannin content? These chemical compounds not only offer resistance to pests but also lend oak its dense and hardy texture.

The Subtle Dance of Molecules and Matter – Nature’s Choreography at Play
- The rich hues of mahogany, a favorite among craftsmen, come from its unique blend of extractives. These chemical compounds, interacting with light, create its sought-after deep reddish-brown shade.
- The compressive strength of wood, vital for structural applications, is influenced by the proportion and arrangement of its cellulose and lignin. In softwoods, this can range from 3,000 to 12,000 psi (pounds per square inch), showcasing the wide diversity in wood’s physical properties due to its chemical makeup.
Dry Wood Properties – The Evolution from Lush to Lustrous
Removing water from wood is akin to metamorphosis, a transformation that turns the familiar into the exquisite.
The Deserted Timber: Wood at its Driest – A Transformation Tale
When wood is reduced to 0% moisture content, it can become 12-15% lighter, making its transportation and handling significantly easier. However, rarely is wood dried to such extents as some moisture is necessary to maintain its workability.

Revealing the Hidden Strengths of Parched Wood – Nature’s Keepsake
- As wood dries, it gains in tensile strength, vital for applications that demand high resistance to stretching. For example, air-dried oak can have a tensile strength of about 10,000 psi, a number that emphasizes its robustness.
- On a personal note, I once worked with a piece of dry cedar wood. Its aroma, a blend of its natural scent and compounds released during drying, was intoxicating. This experience reminded me that even as wood changes in moisture content, it continues to surprise and delight.
Conclusion
For a wood enthusiast like myself, the realm of wood chemistry offers endless revelations. Its complexities not only enrich our understanding but also pave the way for innovations in wood science. As we forge ahead, there’s much to discover, promising an exciting future for wood lovers everywhere.
Related Reads
References
- Eero Sjöström, Wood Chemistry: Fundamentals and Applications, ISBN: 978-0126474817
- R. M. Rowel, Ed., The Chemistry of Solid Wood, ISBN: 0841207968
- R. M. Rowell, Handbook of Wood Chemistry and Wood Composites, Second Edition, ISBN: 9781032099163
- Wood Science and Technology, ISSN: 0043-7719
FAQs
What are the chemical properties of wood?
Wood has several chemical properties that make it a unique material. It is mainly composed of cellulose, lignin, and hemicelluloses. These compounds contribute to its strength, durability, and resistance to chemical reactions.
What is cellulose?
Cellulose is the main chemical component of wood. It is a polysaccharide made up of glucose units linked together. Cellulose provides structural integrity to the cell walls of wood and gives it strength and rigidity.
What is lignin?
Lignin is another important chemical compound found in wood. It is a complex polymer that gives wood its rigidity and resistance to decomposition. Lignin acts as a natural adhesive, binding the cellulose fibers together.
How do chemical properties affect the physical properties of wood?
The chemical composition of wood plays a crucial role in determining its physical properties. The presence of lignin, for example, gives wood its darker color and resistance to decay. Hemicelluloses contribute to the overall flexibility and bending strength of wood.
What are wood extractives?
Wood extractives are chemical compounds found in various parts of wood, such as resins, tannins, and oils. They contribute to the unique aroma, color, and chemical properties of different wood species.
How is wood chemistry analyzed?
Wood chemistry is analyzed through various methods, including chemical analysis. Researchers use techniques such as gas chromatography, mass spectrometry, and nuclear magnetic resonance to identify and quantify the chemical constituents of wood.
What are the main chemical constituents of wood?
The main chemical constituents of wood include cellulose, lignin, and hemicelluloses. These compounds make up the bulk of wood’s chemical composition and contribute to its unique properties.
What is the chemical composition of wood?
The chemical composition of dry wood can vary depending on the species, but on average, wood is approximately 50% cellulose, 25% lignin, and 25% hemicelluloses. Other minor components, such as extractives and inorganic elements, can also be present.
What is hemicellulose?
Hemicellulose is one of the three main components of plant cell walls, alongside cellulose and lignin. Unlike cellulose, which is a highly organized structure, hemicellulose is a random, amorphous structure with little strength. It acts as a filler material in the plant cell wall, filling the spaces between the cellulose fibers and bonding with both the cellulose and lignin, thereby helping to hold the fibers together and providing rigidity to the plant structure.






